NextDose: A web-based Bayesian dose
forecasting tool
Last updated 6 June 2025
Tacrolimus
Tacrolimus is widely used as an immunosuppressant after organ
transplantation. It has been recommended that whole blood concentrations be
interpreted by standardization to those expected with a haematocrit of 45% (Storset, Holford et al. 2014a, Storset,
Holford et al. 2014b, Staatz, Størset et al. 2015).
Body size is best described using predicted fat free mass and theory
based allometric scaling. Concomitant steroid dosing markedly decreases
tacrolimus oral bioavailability (Storset, Holford et al. 2014b, Staatz,
Størset et al. 2015).
Understanding Concentrations
Tacrolimus
concentrations are measured in whole blood. It is measured in whole blood with
concentrations approximately 8,000 times than unbound in plasma (Sikma, Van Maarseveen
et al. 2020). Part of the reason for this is
convenience for the laboratory because concentrations in whole blood are about
20 to 60 times higher than in plasma depending on the haematocrit (Figure
1).
The
distribution and elimination of drugs is determined by unbound concentration,
and it is the unbound concentration that determines both beneficial and adverse
effects. These principles are fundamental in describing and understanding
pharmacokinetics and pharmacodynamics. They apply equally to tacrolimus which
then introduces challenges in interpreting whole blood concentrations.
The
pharmacokinetics of tacrolimus have been described using a theory based
approach to predict plasma concentration under the assumption that unbound
concentration is proportional to plasma concentration (Storset,
Holford et al. 2014b). An essentially linear relationship
between unbound and plasma tacrolimus concentration supports this assumption (Sikma, Van Maarseveen
et al. 2020). The plasma concentration is used
to predict whole blood concentration (erythrocyte bound + plasma) using a
saturable binding model (Jusko,
Piekoszewski et al. 1995).
The
distribution and elimination of tacrolimus is not affected by changes in
erythrocyte binding or changes in haematocrit. Literature reports that claim
changes in haematocrit are associated with changes in clearance are misleading
because tacrolimus elimination is not affected by erythrocyte mass. The
implementation of the theory-based PK model in NextDose uses standardization of
whole blood tacrolimus concentrations to a standard value of 45% which means
changes in PK parameters such as clearance and volume of distribution will be
reflected in the standardized whole blood concentration without being
confounded by changes in haematocrit (Figure
1).
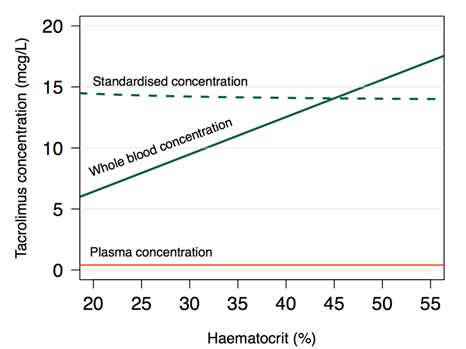
Figure 1 Tacrolimus concentration as a
function of haematocrit (HCT). Red line: Plasma concentration (Cp) (constant at
0.3 mcg/L). Green solid line: Whole blood concentration (Cwb)
calculated from literature values of binding to red blood cells: [Cwb = Cp + Cp × HCT (fraction) × Bmax
/ (Cp + Kd)], where Bmax=418
mcg/L erythrocytes and Kd=3.8 mcg/L plasma [35].
Green dashed line: haematocrit-standardised concentration (Cstd)
(Cstd = Cwb × 45% / HCT%).(Storset, Holford et al. 2014b).
Because of
failure to understand these principles almost all research and clinical use of
tacrolimus concentrations is distorted by not recognizing the misleading
consequences of using un-standardized whole blood concentrations. This
distortion continues today (2024) despite the theory and clinical application
of standardized concentrations having been available since 2014.
Target Concentration
The use of
a trough concentration target is based on tradition but without pharmacological
support. The trough concentration is the lowest concentration during a steady
state dosing interval, but the pharmacological effects are determined by the full time course of concentrations which are necessarily all
higher than or equal to the trough concentration. A more pharmacologically
rational target that captures exposure to all the concentrations causing the
drug effect is the area under the concentration time curve (AUCssDI). Dividing
AUCssDI by the dosing interval (DI) is the average steady state concentration
(Cssavg). Cssavg is independent of the dosing interval and is thus a simple
choice for a target concentration based on pharmacological principles. Nevertheless,
this choice is still rather naïve because it does not account for the time
course of concentration and the delays in the concentration response
relationship but it clearly a step in the right direction away from the
traditional trough concentration.
By default,
NextDose suggests using Cssavg as the target concentration, rather than trough concentration. A CssAvg 15 mcg/L (HCT=45%) is
approximately equivalent to a trough concentration of 7 mcg/L (HCT=33%) (Storset,
Holford et al. 2014b).
There is no pharmacological reason to change the target depending on genotype. The CYP3A4 and CYP3A5 genotypes change exposure (AUCssDI) and thus the average concentration (Cssavg). They do not change the pharmacodynamics of tacrolimus.
NextDose will take care of the CL and F changes when estimating the Bayesian CL and F and use that to predict the dose needed to achieve the target.
A simulation based study compared covariate based dosing (fat free mass) with Bayesian target concentration adapted dosing. Bayesian dosing improved the day 5 Cssavg within an 80-125% acceptable range around the target concentration of 14.2 mcg/L from 37% to 65% (Storset, Holford et al. 2014b).
Figure 2 Simulation study showing improvement in day 5 Cssavg (from (Storset, Holford et al. 2014b)).
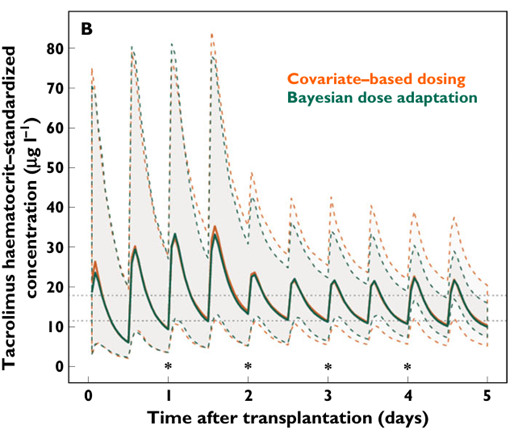
Tacrolimus trough
target concentration attainment was subsequently shown to be improved using
Bayesian forecasting and haematocrit based standardisation of whole blood concentrations (Storset, Asberg
et al. 2015).
CYP3A4 and CYP3A5 Genotypes
In view of increased
interest in genotype based initial dosing of tacrolimus (Khatri,
Felmingham et al. 2024) two CYP3A4 genotypes are included
in NextDose (Figure 3).
Figure 3 NextDose genotypes showing the CYP3A4 and CYP3A5 genotypes which are relevant to tacrolimus.

CYP3A4
normal metaboliser is *1/*1. CYP3A4 poor metaboliser is *22. CYP3A4 poor metabolisers
have a clearance 26% lower than normal metabolisers.
“Because of
the low number of CYP3A5 *1/*1 carriers in the dataset (n = 3), these subjects
were grouped with CYP3A5*1/*3 carriers(n=33) during covariate analysis. CLp was estimated to be 30% higher (ΔOFV −46.0,
P < 0.001) and F 18% lower (ΔOFV −2.9, P = 0.09) in this group
compared with patients not expressing functional CYP3A5 enzyme (*3/*3
carriers). Although an independent effect on F in addition to the effect on CLp was not statistically supported at the significance
level of 0.05 during covariate inclusion, effects on both parameters were
retained because both CLp and F should theoretically
be altered in patients with functional CYP3A5 enzyme in their liver and
intestines.” (Storset,
Holford et al. 2014b)
CYP3A5
normal expresser is *3/*3. CYP3A5 extensive expresser is *1/*1 or *1/*3. CYP3A5
expressers have a 30% increase of CL and 18% decrease in oral bioavailability.
Overall, these two effects of CYP3A5 increased expression decrease tacrolimus
exposure and are used predict the dose required to achieve the target
concentration.
Parameter Estimates and Covariate Effects
The
tacrolimus parameter estimates and covariate effects
are illustrated using data from a child who was given tacrolimus before and
after a kidney transplant. The transplant took place about 5 h after the first
concentration measurement (Figure 4).
Figure 4 Time course of predicted and
observed tacrolimus (HCTstd). Storset2024 model without early
post-transplant effect on oral bioavailability. Note the first 3 concentrations
observed after transplantation are higher than the population prediction
consistent with an early post-transplant effect.

Figure 5 Parameter estimates and covariate effects. Storset2024 model without early transplant effect on oral
bioavailability.
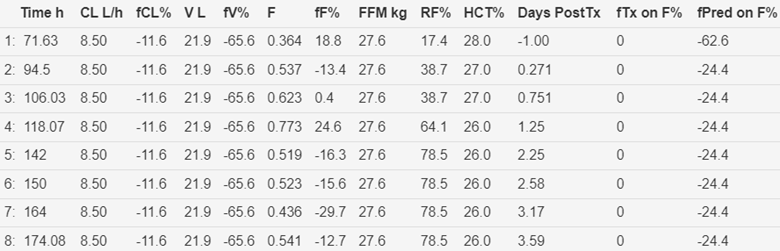
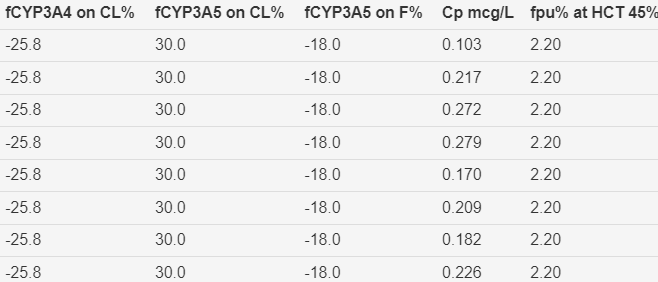
The
fractional early transplant effect on bioavailability (fTx
on F%) is zero because this effect is not included in the Storset2024 model.
The
Storset2024 BTEL model is an extension of the original model for a
post-transplant effect on bioavailability (Storset,
Holford et al. 2014b). Bioavailability has a nominal
value of 1 before transplant. The early post-transplant effect of an increase
in bioavailability in the first two days after transplant is constant over that
period. The post-transplant effect then declines exponentiality with a rapid half life (3 minutes) to reach the nominal value of 1.
There is then a very small linear increase (0.5% /year) in bioavailability as a
function of post-transplant day+2.
See Table 1 and Figure 7 for results
using the Storset2024 BTEL model including time after transplant effects.
Table 1 Parameters and Covariate Effects.
Interpretation comments refer to values in Figure 6.
|
Column |
Description |
Interpretation |
|
Time h |
Time of
observed concentration |
The first
time (71.5 h) is relative to the first dose of tacrolimus |
|
CL L/h |
Empirical Bayes
estimate of whole blood clearance with standard HCT of 45% |
The value
9.06 L/h reflects this is a child (adult value around 17 L/h) |
|
fCL% |
Fractional
difference from group* value for CL reflecting the random between subject
effect |
The
difference is small (2.5%) indicating the covariate effects are doing a good
job of predicting CL |
|
V L |
Empirical
Bayes estimate of whole blood volume of distribution with standard HCT of 45% |
The value 19.2
L reflects this is a child (adult value around 130 L) |
|
fV% |
Fractional
difference from group value for V reflecting the random between subject
effect |
The
difference is large (-68.1%) indicating the covariate effect (size) is doing
a poor job of predicting V |
|
F |
Oral
apparent bioavailability fraction |
The values
less than 1 reflect predictable effects of prednisolone and CYP3A5 expresser
genotype as well as random between subject effects |
|
fF% |
Fractional
difference from group value for F reflecting the random between subject
effect |
The
differences are have a modest (-26.3% to -31.5%)
indicating the covariate effects are doing a reasonable job at predicting F |
|
FFM kg |
Predicted
fat free mass based on total body mass, height, sex and postnatal age |
FFM is used
as the allometric mass for scaling CL and V |
|
RF |
Renal
function |
Not used in
the PK model but reflects improvement following transplant |
|
HCT% |
Observed
haematocrit |
HCT is used
to standardize tacrolimus concentrations |
|
Days Post
Tx |
Days following
the time of transplant |
The -1
value means the observed concentration was obtained prior to the transplant
(not days before transplant). Days post-transplant are
used to predict the early increase in bioavailability when days are greater
than zero and less than 2. |
|
fTx
on F% |
Fractional
difference from group value for early transplant effect on bioavailability
reflecting the random between occasion effect |
Oral bioavailability has a nominal
value of 1. The Storset2024 BTEL model group estimate is a 2.57 x increase in
oral bioavailability between 0 and 2 days post-transplant This is consistent
with (Storset,
Holford et al. 2014b). The Ftx
on F% value of -47% reflect the random between subject effect indicating this
subject the bioavailability appears only to have increased by about 20.8%
(0.47 x 2.57-1) during the first 2 days post-transplant. After 2 days there
are only small differences in F relative to the nominal value of 1. fTx on F% value is
always 0 for the Storset2024 model because a post-transplant effect is not
included. |
|
fPred
on F% |
Fractional
difference from group value for prednisolone effect on bioavailability
reflecting the random between occasion effect |
For the 500
mg/day prednisolone dose there is a large difference (-60.8%) from the group
effect perhaps because the effect was estimated in adults. For the 10 mg/day
dose the difference is modest (-26.5.4%) indicating is doing a reasonable job
of predicting F. |
|
fCYP3A4 on
CL% |
Genotype
prediction of effect on clearance |
CL is 25.8%
lower for a subject with a poor metaboliser
genotype |
|
fCYP3A5 on
CL% |
Genotype
prediction of effect on clearance |
CL is 30%
higher for a subject with an expresser genotype |
|
fCYP3A5 on
CL% |
Genotype prediction
of effect on bioavailability |
F is 13.3%
lower for a subject with an expresser genotype |
|
Cp mcg/L |
Individual
prediction of tacrolimus plasma concentration |
The Storset
model is based on predicted plasma concentrations. The Kd
for binding to red cell mass is 3.8 mcg/L (Jusko, Piekoszewski et al. 1995). |
|
fpu%
at HCT 45% |
Individual
prediction of tacrolimus plasma unbound to red cell mass |
The
fraction of plasma unbound and observed HCT is used to predict whole blood
concentrations for comparison with observed whole blood concentrations. |
* The group
value is the parameter value after incorporating the predictable covariate
effects without any random effects. In older literature it might be called the
“typical value”.
Figure 6 Time course of predicted and
observed tacrolimus (HCTstd). Storset2024 BTEL model including early
transplant effect on oral bioavailability.

The
predicted increase in bioavailability in the 2 days following transplant is
reflected in the 4 high peak population concentrations. The individual
predictions are lower because of the estimated random effect for this subject.
Figure 7 Parameter estimates and covariate
effects. Storset2024 BTEL
model including time after transplant effect on oral bioavailability.
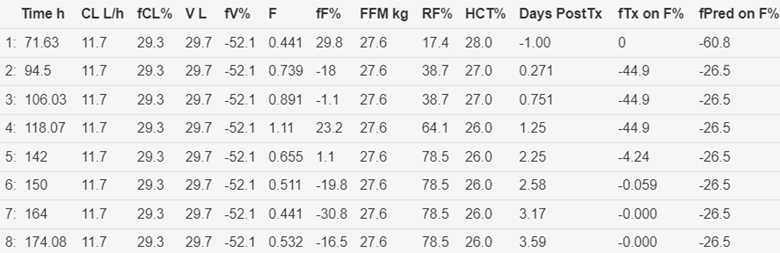
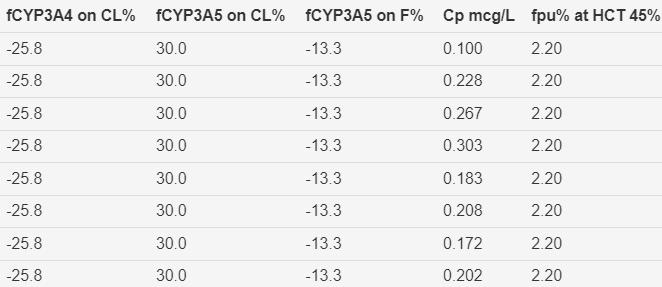
Figure 8 Time course of predicted and observed tacrolimus
(HCTstd). Storset2024 model without early transplant effect on oral
bioavailability and without first 4 tacrolimus observations in the
peri-transplant period.
Figure 9 Parameter estimates and covariate effects. Storset2024 model without
early transplant effect on oral bioavailability and without first 4
tacrolimus observations in the peri-transplant period.
Figure 10 Time course of predicted and
observed tacrolimus (HCTstd). Storset2024 BTEL model including
early transplant effect on oral bioavailability but without first 4
tacrolimus observations in the peri-transplant period.
Figure 11 Parameter estimates and
covariate effects. Storset2024
BTEL model including early transplant effect on
oral bioavailability but without first 4 tacrolimus observations in the
peri-transplant period.
The
Storset2025 BTEL model includes an effect for the time course of tacrolimus CL
after liver transplant. This is based on the Nanga (2019) meta-analysis (Nanga, Doan et
al. 2019). This assumes CL immediately after
transplant (day 0) is 50% of the eventual value when the transplanted liver function
is stable. The stable function CL is 76% of the CL for a similar patient following
a kidney transplant. The time course of
recovery of a transplanted liver reaches 75% of stable function around 6 days post transplant and approaches stable function around 10
days post-transplant.
References
Jusko, W. J., W. Piekoszewski, G. B. Klintmalm, M. S. Shaefer, M. F. Hebert, A. A. Piergies, C. C. Lee, P. Schechter and Q. A. Mekki (1995). "Pharmacokinetics of tacrolimus in liver transplant patients." Clin Pharmacol Ther 57(3): 281-290.
Khatri, D., B. Felmingham, C. Moore, S. Lazaraki, T. Stenta, L. Collier, D. A. Elliott, D. Metz and R. Conyers (2024). "Evaluating the evidence for genotype-informed Bayesian dosing of tacrolimus in children undergoing solid organ transplantation: A systematic literature review." British Journal of Clinical Pharmacology Early View(n/a).
Nanga, T. M., T. T. P. Doan, P. Marquet and F. T. Musuamba (2019). "Toward a robust tool for pharmacokinetic-based personalization of treatment with tacrolimus in solid organ transplantation: A model-based meta-analysis approach." British Journal of Clinical Pharmacology 85(12): 2793-2823.
Sikma, M. A., E. M. Van Maarseveen, C. C. Hunault, J. M. Moreno, E. A. Van de Graaf, J. H. Kirkels, M. C. Verhaar, J. C. Grutters, J. Kesecioglu, D. W. De Lange and A. D. R. Huitema (2020). "Unbound Plasma, Total Plasma, and Whole-Blood Tacrolimus Pharmacokinetics Early After Thoracic Organ Transplantation." Clinical Pharmacokinetics 59(6): 771-780.
Staatz, C. E., E. Størset, T. K. Bergmann, S. Hennig and N. Holford (2015). "Tacrolimus pharmacokinetics after kidney transplantation – Influence of changes in haematocrit and steroid dose." British Journal of Clinical Pharmacology: DOI: 10.1111/bcp.12729.
Storset, E., A. Asberg, M. Skauby, M. Neely, S. Bergan, S. Bremer and K. Midtvedt (2015). "Improved Tacrolimus Target Concentration Achievement Using Computerized Dosing in Renal Transplant Recipients--A Prospective, Randomized Study." Transplantation 99(10): 2158-2166.
Storset, E., N. Holford, S. Hennig, T. K. Bergmann, S. Bergan, S. Bremer, A. Asberg, K. Midtvedt and C. E. Staatz (2014b). "Improved prediction of tacrolimus concentrations early after kidney transplantation using theory-based pharmacokinetic modelling." Br J Clin Pharmacol 78(3): 509-523.
Storset, E., N. Holford, K. Midtvedt, S. Bremer, S. Bergan and A. Asberg (2014a). "Importance of hematocrit for a tacrolimus target concentration strategy." Eur J Clin Pharmacol 70(1): 65-77.
Copyright All rights reserved |
Developed by Sam Holford & Nick Holford 2012-2024